The definition and classification of infant formula for special medical purposes are as follows.
Q&A on Product Formula Registration of IF III
The Q&As on product formula registration of infant food III are as follows.

The definition and classification of infant formula for special medical purposes are as follows.

The Q&As on product formula registration of infant food III are as follows.

To ensure the success of the fourth China International Import Expo, the General Administration of Customs has outlined its initiatives for streamlining customs clearance and promoting trade facilitation. It recently released the Customs Cl

This year’s The State of Food Security and Nutrition in the World(SOFI) summarizes the first global assessment of food insecurity and malnutrition for 2020 and offers some indication of what hunger and malnutrition would look like by 2030

A growing number of young Chinese who buy near-expired food at reduced prices, a practice that has been spurred by a more rational consumption concept and the adoption of the anti-food-waste law in the country in April.

Excerpts from Q&A on Product Formula Registration of IF are as follows.

In order to further deepen the reform of “decentralization, regulation and service”, and optimize the business environment, the GACC has implemented a notification and commitment system for certification items on imported dairy products

With the booming market of plant-based food in China, industry is looking for regulatory guidance. Some guidance came in late 2020 when the Chinese Institute of Food Science and Technology (CIFST) published the first voluntary group standar

Special marking contents required on such as canned complementary food for infants and young children and food supplement

From June 23 to 25 , Healthplex Expo 2021 and HNC 2021 were successfully held at the Shanghai National Exhibition Convention Centre. As the official partner of this event, Antion conducted a series of regulatory activities at the event. Foo

In order to further guide applicants to scientifically carry out formula R&D and trial production, and to standardize the application of registration documents, the SAMR has compiled and formed the Q&A on Product Formula Registration of Infant and You

Special marking contents required on infant formula, older infants and young children formula, cereal-based complementary foods for infants and young children

On June 15, 2021, the SAMR issued the Announcement on Strengthening the Quality and Safety Supervision of Solid Beverages (Draft for Comments) (Hereinafter referred to as the Draft), the deadline for comments is July 14, 2021. The Draft req

How to mark nutritional fortification substances when registering formula of infant food (IF)? The names of food raw materials and food additives in the ingredients list shall be consistent with the formula composition. Because each nutriti

Special marking contents required on food for special medical purpose and infant food for special medical purpose

On June 4, 2021, the SAMR issued the Announcement of the SAMR on Supplementing the Marking of Edible Duration on the Labeling of Low Temperature and Short Shelf Life Dairy Products (Draft for Comments). From October 1, 2022, if producing pa

How to calculate and label the fat in catering food? The fat in catering food includes the fat from food raw materials and the fat added during the cooking process. The fat content in the dishes can be calculated based on the amount of raw

How to mark compound ingredients when registering formula of IF food? The ingredients list shall list the names and use amount of all food raw materials and food additives used, and the use amount of the ingredients marked on the ingredient

Catering service providers in Beijing who encourage, mislead or force consumers to order too much food may face fines of up to 10,000 Yuan ($1,570), according to a regulation passed by the municipal legislature on Thursday. The regulation,

On June 2, 2021, the NHC publicly solicited opinions on 23 national food safety standards (draft for comments) including Cream, Butter and Anhydrous Milkfat, Tea, Herbal Tea and Plant Protein Peptides for Food Processing. The deadline for c

Recently, the Guangdong Administration for Market Regulation issued a notice on the verification and disposal of a batch of unqualified foods. The notice showed that a batch of goat milk powder imported by Health Happiness Group failed to p

The following content is the answer of the NHC to the general concern about infant formula food.

According to the monthly notice of non-entry foods by GACC, 157 batches of imported foods were found unqualified and not allowed to enter China in April 2021 . The information shows that non-entry foods came from 29 countries or regions inc

NHNE 2021 Successfully Ended! More Events Please Follow Us From May 18 to 20, the 22nd SIAL China International was successfully held at the Shanghai New International Expo Center. Antion held the New Trend of Nutrition and Health Food Foru

Recently, the law enforcement officers of Nanjing Administration for Market Supervision found a number of breads in the kitchen waste bin of a bakery, and all bread were discarded because their sizes did not meet the finished product requir

Case| Abbott was Fined 9 Million Due to Vanillin Detected in IF A few days ago, the Shanghai Administration for Market Regulation imposed administrative penalties on Abbott Trading (Shanghai) Co., Ltd., accused it of “production and opera

The top legislature on Thursday adopted a new law on preventing food waste to offer legal backing to the countrys efforts to safeguard food security and promote traditional virtue of thrift. The law, passed at a session of the Standing Comm

In recent years, consumers’ shopping behaviors and concepts have undergone tremendous changes. Under the general trend of domestic consumption upgrading, the popularity of organic food continues to rise.

If they are used as nutritional fortification substances, the use should comply with the requirements of GB 14880

If the ingredients of health food can also be used for general food, the health food is allowed to share production sites and facilities with general food.

Formulated milk powder means powder product that produced from raw bovine milk or ovine milk or its processed products as the major ingredient, with addition of other ingredients, with or without addition of food additives and nutritional fo

If manufacturers intend to add species into infant formula foods, the viable count in products should be not less than 106CFU/g (mL), and the used species (strains No.) should conform with the List of Species that can be Used in Infant Food.

GACC recently announced the consumption data of infant formula milk powder imports in the first quarter, which shows that from January to March 2021, the domestic import of 61,000 tons of infant formula milk powder, down 17.6% compared to th

From May 12 to 14, NHNE 2021 China International Natural Health Nutrition Expo was successfully held at the Shanghai National Exhibition Convention Centre. As the official partner of this event, Antion conducted a series of regulatory activ

Recently, Shanghai Administration for Market Regulation announced the “first batch of typical cases of false and illegal advertisements in 2021”. Among them, Shanghai Caorun Biotechnology Co., Ltd. was fined 150,000 Yuan for publishing

The health food industry is an important part of the health industry. In recent years, with the enhancement of public health awareness, China’s health food industry has been developing rapidly, with the market size expected to reach 330.7

although both “live probiotic-type solid beverage” and “probiotic health food” are foods containing live probiotic, one is general food and the other is health food. They implement different management regulations and requirements an

Recently, the State Administration for Market Regulation (SAMR) has issued the 2021 Legislative Work Plan (hereinafter referred to as the Plan ), and made arrangements for the legislative work in 2021. The Plan shows that the SAMR will clos

On March 26, NHC issued a Letter of Soliciting Opinions on 12 National Food Safety Standards including National Food Safety Standard Standard for Uses of Food Additives (Draft for Comments) . These standards include 11 standards for food ad

In order to do a good job in the product formula registration of infant and young children formula milk powder after the issuance of new national standards, the announcement regarding registration matters is as follows

On March 18, 2021, the NHC issued the National Food Safety Standard Cheese (GB 5420-2021), which will come into effect on November 22, 2021.

CBEC platform operators should truthfully determine the nature and type of commodities when selling overseas commodities, and should bear corresponding responsibilities if the translation of commodity information in a misleading manner cause

On March 18, 2021, the NHC and the SAMR jointly issued Announcement No. 3 of 2021, releasing 50 new national food safety standards and 4 amended documents , including 3 nutrition and special dietary food standards such as Infants Formula (G

In On April 12, 2021, the General Administration of Customs announced Order No. 248 of Regulations of the People's Republic of China on the Registration and Administration of Overseas Manufacturers of Imported Food (hereinafter referred to a

On April 12, 2021, the General Administration of Customs announced Order No. 249 of Administrative Measures for Import and Export Food Safety (hereinafter referred to as Measures), which will be implemented on January 1, 2022.

New Standards put forward more stringent requirements for product quality requirements. Nutrient indicators have been adjusted. New Standards sets maximum values for all nutrients of older infants formula. And refine product standards and split the cu

If you are interested, welcome to contact us!

An imported food label did not indicate the name, address, and contact information of the domestic agent, which violated the requirements for imported food in the Food Safety Law. On the other hand, this product is general food, illegally ad

According to Article 7 of Administration Measures on Formula Registration of Milk Powdered Formula for Infant and Young Children , applicants shall be manufacturers that plan to produce and sell infant formula milk powder within the territo

As the epidemic promoted the industry reshuffle, further intensified the market stock competition, the second formula registration and the landing of the new national standard will make the industry further reshuffle, industry competition is

What are the requirements for imported food in the Food Safety Law ? The article 92 of Food Safety Law indicates that imported food, imported food additive, and imported food-related product should comply with China national food safety sta

On February 20, 2021, the SAMR issued the Available Auxiliary Ingredients and the Use Regulations for Filing Health Food (2021 Edition) and Dosage Form and Technical Requirements of Filing Health Food (2021 Edition) , which included powder

The label of imported prepackaged food should meet the requirements of 3.8.2 in GB 7718-2011 National food safety Standard General Standard for the Labeling of Prepackaged Foods regarding the one-to-one correspondence between Chinese and fo

On February 18, 2021, China National Centre for Food Safety Risk Assessment (CFSA) solicited public opinions on 10 new varieties of food additives, including 9 processing aid-enzyme preparations for food industry and 1 sweetener with expande

Filling health food using vitamins and minerals as ingredients included in the Catalogue can be made as gummy form.

NMN in China has not obtained any licenses such as medicine, health food, food additive and novel food, thus, it cannot be produced and sold as food.

On February 8, 2021, NHC issued a Letter of Soliciting Opinions on National Food Safety Standards including National Food Safety Standard Nutritional Fortification Substances in Foods L-Carnitine Tartrate (Draft for Comments) . These standa
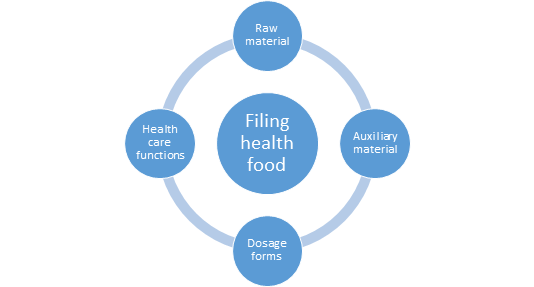
Technical Requirements indicates the specific provisions on the available dosage forms, auxiliary materials and technical requirements for Coenzyme Q10, Fish oil, Sporoderm-broken of ganoderma lucidum spores powder, Spirulina and Melatonin,

In the year of 2020, China government revealed several regulations and drafts for comments you are interested. Antion analyzed and interpreted relevant regulations and drafts.

The Draft clarifies these Regulations apply to the registration, supervision, and management of overseas manufacturers, processors, and storage facilities that export foods to China, excluding production, processing, and storage of food additives and
Coenzyme Q10, Melatonin, Fish oil, Spirulina, and Sporoderm-broken of Ganoderma Lucidum Spores Powder

Including R&D report, qualification certificate of applicant, product formula, proof materials for R&D capabilities, production capabilities, and inspection capabilities, etc.

In the year 2020, China government revealed several regulations and drafts for comments about health products you are interested in. Antion pulled a list for those articles and some other related information that worth reading again.

2020 Industry Report for Infant Formula Milk Powder is coming out, which is published by Antion every year. The main contents of the report include the development status of infant formula milk powder market, summary of infant formula milk p

The height of the numbers should be greater than or equal to 1.8mm.

When the nutritional need of certain people cannot be met through ordinary meals or daily meals, FSMP can be used as an effective way of nutritional supplementation and play a role in nutritional support. The following is a brief description

Frustrated at the overuse of the term ‘natural’ to define food products and ingredients that ‘do not always match consumers’ expectations’, Safe Food Advocacy Europe is calling on the European Union to develop a legal definition.

On December 30, 2020, the SAMR announced the Dairy Products Quality and Safety Improvement Action Plan (hereinafter referred to as the Plan) to further improve the quality and safety of dairy products and promote the high-quality development

Fruiting body of Isaria cicadae Miq.(artificially cultured), sodium hyaluronate, and Lactobacillus kefiranofaciens subsp. kefiranofaciens, were approved.

On January 6, 2021, Antion successfully held a live streaming course of 2020 Food Regulations Roundup

China customs has implemented a market access system for imported infant and young children formula milk powderand has strict inspection and quarantine supervision requirements.

l Overview of Annual Food Regulation Update l Interpretation of Key Food Regulations l Cases of Key Food Regulations l Trend Analysis of Food Regulations