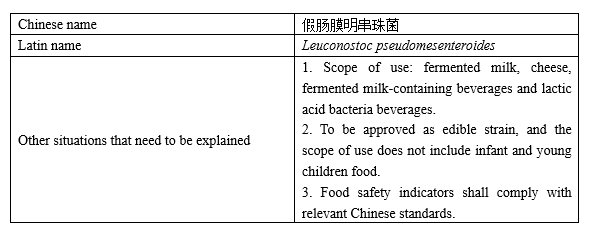
Recently, China National Centre for Food Safety Risk Assessment (CFSA) publicly solicits opinions on "three novel foods", including the novel food ingredient—Leuconostoc pseudomesenteroides, 4 new varieties of enzyme preparations for food industry including aminopeptidase, processing aids for food industry with the scope of use expanded—polydimethyl siloxane and emulsion and magnesium stearate, as well as new varieties of food nutritional fortification substances—L-alanine and L-arginine hydrochloride. The deadline for comments is July 29, 2022.
Novel food ingredient
4 new varieties of enzyme preparations for food industry
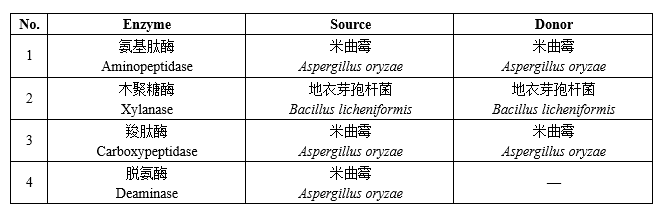
The quality specification requirements of enzyme preparations for food industry shall comply with the provisions of the National Food Safety Standard Food Additives Enzyme Preparations for Food Industry (GB 1886.174).
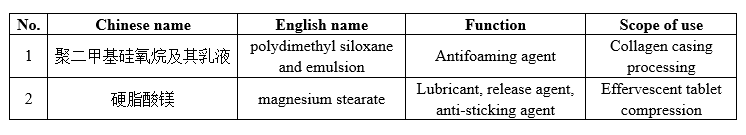
2 processing aids for food industry with the scope of use expanded
2 new varieties of food nutritional fortification substances
1. Chinese name: L-丙氨酸
English name: L-Alanine
Function classification: food nutritional fortification substance
Usage and scope of use

2. Chinese name: L-盐酸精氨酸(又名L-精氨酸盐酸盐)
English name: L-Arginine Hydrochloride
Function classification: food nutritional fortification substance
Usage and scope of use

If you are interested in the application of three novel food", please feel free to contact us!
Hongtao Fei
Tel: 010-51301566
email: feiht@antion.net
Source: CFSA
Note: This article is compiled by Antion, please indicate our source if reprint it.