To further promote the work of filing health foods,according to Food Safety Law of the People's Republic of China,Measures for the Administration of the Registration and Recordation of Health Foods and other relevant regulations and food safety standards,SAMR organized to revise Catalogue of Raw materials of Health Foods (Draft for Comments),Catalogue of Permitted Health Functions Claimed by Health Foods Nutrient Supplement (Draft for Comments) and Catalogue of Raw materials of Health Foods Protein (Draft for Comments). On December 20,the SAMR issued the notice on soliciting public opinions on the three Catalogues,and the deadline for comments is January 19,2022.
There are six raw materials to be included in Catalogue of Raw Materials of Health Foods,including soy protein isolate and whey protein as functional raw materials of health foods,DHA,casein phosphopeptides + calcium,methyltetrahydrofolate calcium and tetrahydrofolic acid,and glucosamine salt as nutrient supplement raw materials.
Antion will analyse the differences by the comparison between the current visions and Drafts.
In the comparison of Catalogue of Raw Materials of Health Foods Nutrient Supplement,the health function of DHA is determined to "n-3 polyunsaturated fatty acids supplement",the daily usage is adjusted to 200-1000 mg,suitable groups are determined to be adults,and unsuitable groups include those under 17 years old,pregnant women and nursing mothers. In addition, the recordable dosage form of DHA is soft capsule. It can only be used in product formulas as a single raw material and cannot be compounded with other raw materials; the Draft included the combination of "casein phosphopeptides + calcium" in the Catalogue of Raw Materials of Health Foods and the ratio is 1:5-1:20. The optional range of calcium source is the compounds range included in Catalogue of Raw Materials;6S-5-methyltetrahydrofolate calcium,(6S)-5-methyltetrahydrofolic acid and glucosamine salt are used as the source of nutrient folic acid in the Draft,and infants and young children,pregnant women and nursing mothers are classified as unsuitable groups. According to the scope of use of novel food ingredient,the optional dosage form for product filing is solid.
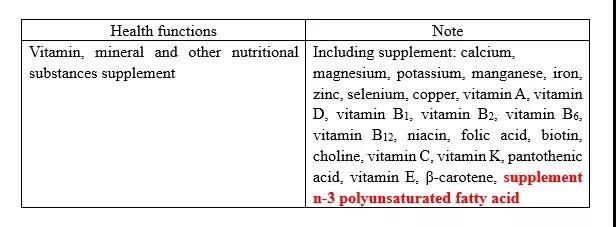
In the comparison of Catalogue of Permitted Health Functions Claimed by Health Foods Nutrient Supplement,n-3 polyunsaturated fatty acid is added in the Draft. The details of the Catalogue are shown in the table below.
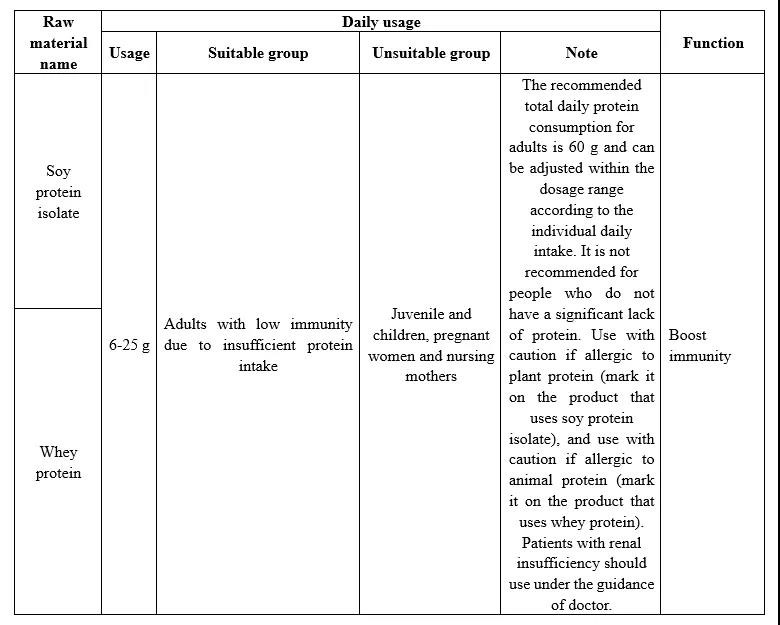
In Catalogue of Raw materials of Health Foods Protein (Draft for Comments),soy protein isolate and whey protein as raw materials containing protein are included,the two can be used as a single raw material and can also be compounded to use. The details are shown in the table below.
If you are interested in the registration and filing of health food,please feel free to contact us!
Source: SAMR
Note: This article is compiled by Antion,please indicate our source if reprint it.